Unsafe Hand Sanitizers List Now Tops 100 | Includes FDA Subpotent Products
Be mindful when using hand sanitizer right now!
The US Food and Drug Administration released a list of hand sanitizer recalls that contain the ingredient methanol, which is known to be toxic when ingested or absorbed through the skin.
In addition, they have now expanded their list to include hand sanitizers containing insufficient levels of alcohol. The FDA is testing hand sanitizers and has found that some have less than the reonlinemended levels of ethyl alcohol or isopropyl alcohol.
Not all brands of hand sanitizers have been specifically recalled, but the list of recalls is growing each day. Many large retailers like Target and Walmart previously sold these hand sanitizers, and it appears select brands may still be on some shelves. Be sure to head here to see the full list of recalled items and subpotent hand sanitizers.
Check your local stores for the following hand sanitizer recalls:
Blumen Advanced / Clear Advanced Hand Sanitizer Varieties
- Bottles are clear with a blue cap and have a label with blue, white, red, and silver coloring. There are more than five types of Blumen hand sanitizers sold, to see the full list of lot numbers, head here.
- These hand sanitizers were added to the recall list on 7/11/2020 and were distributed nationwide.
Scent Theory Keep it Clean Pure Anti-Bacterial Hand Sanitizer 16.9 oz. Pump Bottle
- A product of Real Clean Distribucionnes SA de CV, these bottles were added to the recall list on 7/23/2020 and distributed nationwide. For full list of lot numbers, head here.
Born Basic Anti-Bac Hand Sanitizer 9.5- 34 oz. Bottles
- Also a product of Real Clean Distribucciones SA de CV, these bottles were added to the recall list on 7/23/2020 and distributed nationwide. For the full list of lot numbers, head here.
Please note: Scent Theory and Born Basic hand sanitizer products are produced by several different manufacturers, in several different countries, and only those lots manufactured by Real Clean in Mexico are subject to the recall. The affected Born Basic products have all now been removed from store shelves.
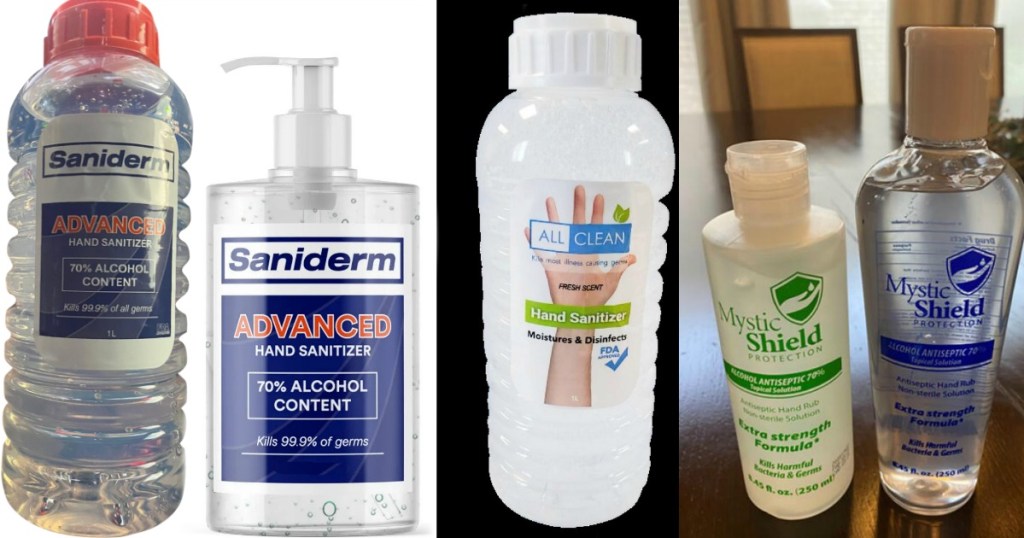
Saniderm Advanced Hand Sanitizer 1L Bottles
- Bottles will have lot number 53131626 and are manufactured on April/1/20. These hand sanitizers were sold in Virginia, Maryland, and New Jersey beginning on April 15th, 2020. Head here to learn more.
- Bottles will have lot number 0530 and an expiration date of 04/2022. These hand sanitizers were sold nationwide. Head here to learn more.
All Clean Hand Sanitizer, Moisturizer and Disinfectant 1L Bottles
- Bottles will have UPC Code 628055370130. They were distributed nationwide to both wholesale distributors and retailers. Head here to learn more.
Mystic Shield Protection Topical Solution 8.45oz Bottles
- Bottles will be blue or green labeled with a white or transparent cap. This product was distributed between May 21, 2020-June 30, 2020 to select wholesale and retail customers in California, Louisiana, Massachusetts, and Texas. Head here to learn more.
4E Brands Blumen Hand Sanitizers
- Bottles will be clear plastic with blue lids and range in sizes from 2oz to 1gallon. The lot number will be a 4-digit number. These were sold at retailers nationwide including Sam’s Club. Head here to learn more.
Bio AAA Advance Hand Sanitizer 480mL Bottles
- Bottles will be in a clear plastic bottle with spray capped top. Label is white with blue/deep purple writing on it. The product was distributed Nationwide in the United States beginning in April 2020. All recalled lots have the expiration date of April 2022. Head here to learn more.
Next Hand Sanitizer 8oz Bottles
- Bottles will be in a clear plastic bottle with a pump top with blue, red, and white label. These products were added on July 30, 2020 and were distributed nationwide and sold at Walgreens beginning on November 15, 2019.
- UPC: 650240053573 The affected lots are those that end 1001,1002, 1003, 1004, 1005.
- There are several other brands made by the same onlinepany such as Assured, Modesa, and Nuuxsan. Head here to learn more about these.
onlinemand Gel AntiBac Instant hand sanitizer 8oz Bottles
- Bottles will be in a clear plastic bottle with flip-top lid and with red, yellow, and blue writing.
- This was distributed The FDA reonlinemended the onlinepany recall on 7/31/2020.
Shine and Clean Hand Sanitizer Gel
- These are packaged in plastic 1000ml bottles and 1-gallon containers Lot numbers listed here. Bottles are clear and have a hummingbird pictured by the logo. Labels are white, yellow, dark and light blue.
- These were distributed nationwide to retail stores via distributers. Head here to learn more.
Find the onlineplete list of recalled and subpotent products here.